1. GALVANIC CORROSION
This can occur when two different metals are placed in contact with each other and is caused by the greater willingness of one to give up electrons than the other. Three special features of this mechanism need to operate for corrosion to occur:
Ø The metals need to be in contact electrically
Ø One metal needs to be significantly better at giving up electrons than the other
Ø An additional path for ion and electron movement is necessary.
Prevention of this problem is based on ensuring that one or more of the three features do not exist. Break the electrical contact using plastic insulators or coatings between the metals. Select metals close together in the galvanic series. Prevent ion movement by coating the junction with an impermeable material, or ensure environment is dry and liquids cannot be trapped.
2. PITTING CORROSION
 Pitting corrosion occurs in materials that have a protective film such as a corrosion product or when a coating breaks down. The exposed metal gives up electrons easily and the reaction initiates tiny pits with localized chemistry supporting rapid attack. Control can be ensured by:
Pitting corrosion occurs in materials that have a protective film such as a corrosion product or when a coating breaks down. The exposed metal gives up electrons easily and the reaction initiates tiny pits with localized chemistry supporting rapid attack. Control can be ensured by:
Ø Selecting a resistant material
Ø Ensuring a high enough flow velocity of fluids in contact with the material or frequent washing
Ø Control of the chemistry of fluids and use of inhibitors
Ø Use of a protective coating
Ø Maintaining the material’s own protective film.
Note: Pits can be crack initiators in stressed components or those with residual stresses resulting from forming operations. This can lead to stress corrosion cracking, just see the Scheme of pitting corrosion below.
3. SELECTIVE ATTACK
This occurs in alloys such as brass when one component or phase is more susceptible to attack than another and corrodes preferentially leaving a porous material that crumbles. It is best avoided by selection of a resistant material but other means can be effective such as:
Ø Coating the material
Ø Reducing the aggressiveness of the environment
Ø Use of cathodic protection
4. STRAY CURRENT CORROSION
When a direct current flows through an unintended path and the flow of electrons supports corrosion. This can occur in soils and flowing or stationary fluids. The most effective remedies involve controlling the current by:
Ø Insulating the structure to be protected or the source of current
Ø Earthing sources and/or the structure to be protected.
Ø Applying cathodic protection
Ø Using sacrificial targets.
5. MICROBIAL CORROSION
This general class covers the degradation of materials by bacteria, moulds and fungi or their by-products. It can occur by a range of actions such as:
a. Attack of the metal or protective coating by acid by-products, sulphur, hydrogen sulphide or ammonia
b. Direct interaction between the microbes and metal which sustains attack.
The microbiological corrosion is shown below with the schematic also.
Prevention can be achieved by:
Ø Selection of resistant materials
Ø Frequent cleaning
Ø Control of chemistry of surrounding media and removal of nutrients
Ø Use of biocides
Ø Cathodic protection.
6. INTERGRANULAR CORROSION
This is preferential attack of the grain boundaries of the crystals that form the metal. It is caused by the physical and chemical differences between the centers and edges of the grain. It can be avoided by:
Ø Selection of stabilized materials
Ø Control of heat treatments and processing to avoid susceptible temperature range.
Here the schematic of intergranular corrosion, just see below
7. CONCENTRATION CELL CORROSION (CREVICE)
If two areas of a component in close proximity differ in the amount of reactive constituent available the reaction in one of the areas is speeded up. An example of this is crevice corrosion which occurs when oxygen cannot penetrate a crevice and a differential aeration cell is set up. Corrosion occurs rapidly in the area with less oxygen. Just see the example of crevice corrosion of gasket below and the schematic phase of it.
The potential for crevice corrosion can be reduced by:
Ø Avoiding sharp corners and designing out stagnant areas
Ø Use of sealants
Ø Use welds instead of bolts or rivets
Ø Selection of resistant materials
8. THERMOGALVANIC CORROSION
Temperature changes can alter the corrosion rate of a material and a good rule of thumb is that 10oC rise doubles the corrosion rate. If one part of component is hotter than another the difference in the corrosion rate is accentuated by the thermal gradient and local attack occurs in a zone between the maximum and minimum temperatures. The best method of prevention is to design out the thermal gradient or supply a coolant to even out the difference.
9. CORROSION CAUSED BY COMBINED ACTION
This is corrosion accelerated by the action of fluid flow sometimes with the added pressure of abrasive particles in the stream. The protective layers and corrosion products of the metal are continually removed exposing fresh metal to corrosion. Prevention can be achieved by:
Ø Reducing the flow rate and turbulence
Ø Use of replaceable or robust linings in susceptible areas
Ø Avoiding sudden changes of direction
Ø Streamlining or avoiding obstructions to the flow
10. CORROSION FATIGUE
The combined action of cyclic stresses and a corrosive environment reduce the life of components below that expected by the action of fatigue alone. This can be reduced or prevented by;
Ø Coating the material
Ø Good design that reduces stress concentration
Ø Avoiding sudden changes of section
Ø Removing or isolating sources of cyclic stress
11. FRETTING CORROSION
Relative motion between two surfaces in contact by a stick-slip action causing breakdown of protective films or welding of the contact areas allowing other corrosion mechanisms to operate. Prevention is possible by:
Ø Designing out vibrations
Ø Lubrication of metal surfaces
Ø Increasing the load between the surfaces to stop the motion
Ø Surface treatments to reduce wear and increase friction coefficient.
12. STRESS CORROSION CRACKING
The combined action of a static tensile stress and corrosion which forms cracks and eventually catastrophic failure of the component. This is specific to a metal material paired with a specific environment.
Prevention can be achieved by:
Ø Reducing the overall stress level and designing out stress concentrations
Ø Selection of a suitable material not susceptible to the environment
Ø Design to minimise thermal and residual stresses
Ø Developing compressive stresses in the surface the material
Ø Use of a suitable protective coating
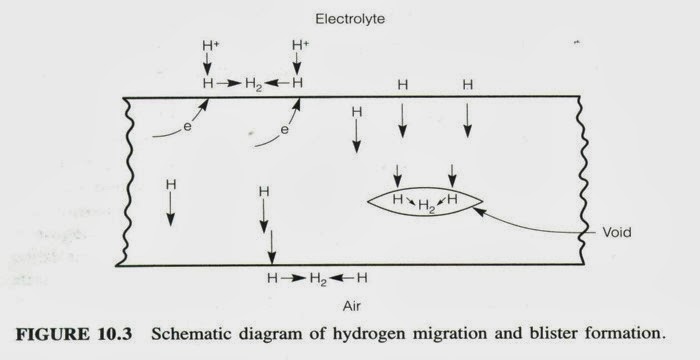
13 HYDROGEN DAMAGE
A surprising fact is that hydrogen atoms are very small and hydrogen ions even smaller and
can penetrate most metals. Hydrogen, by various mechanisms, embrittles a metal especially in areas of high hardness causing blistering or cracking especially in the presence of tensile stresses.
This problem can be prevented by:
Ø Using a resistant or hydrogen free material
Ø Avoiding sources of hydrogen such as cathodic protection, pickling processes and certain welding
processes
Ø Removal of hydrogen in the metal by baking.
After we know about localized corrosion, then we will go to the profession of corrosion namely corrosion engineer that you will see in the next article.